INTRO
Our research is guided by a simple yet profound goal: to better understand human diseases.
The human body maintains remarkable stability despite constant changes in the environment. This stability—known as homeostasis—depends on tissues and organs that continuously communicate, repair, and adapt. When these processes fail, disease emerges.
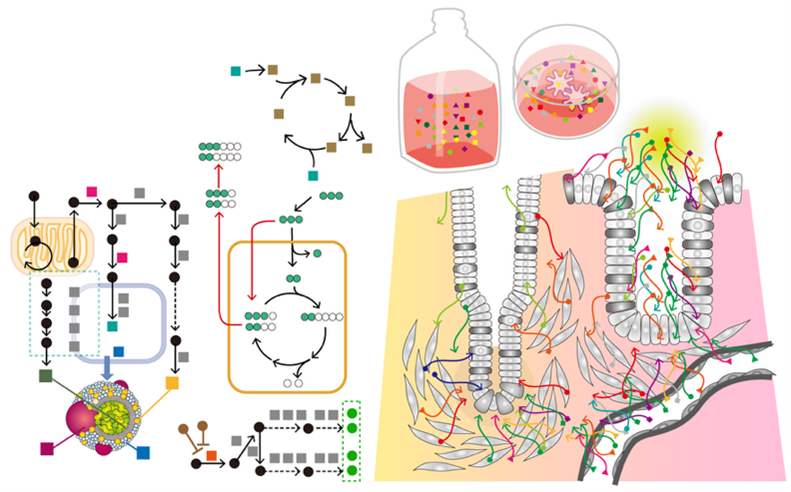
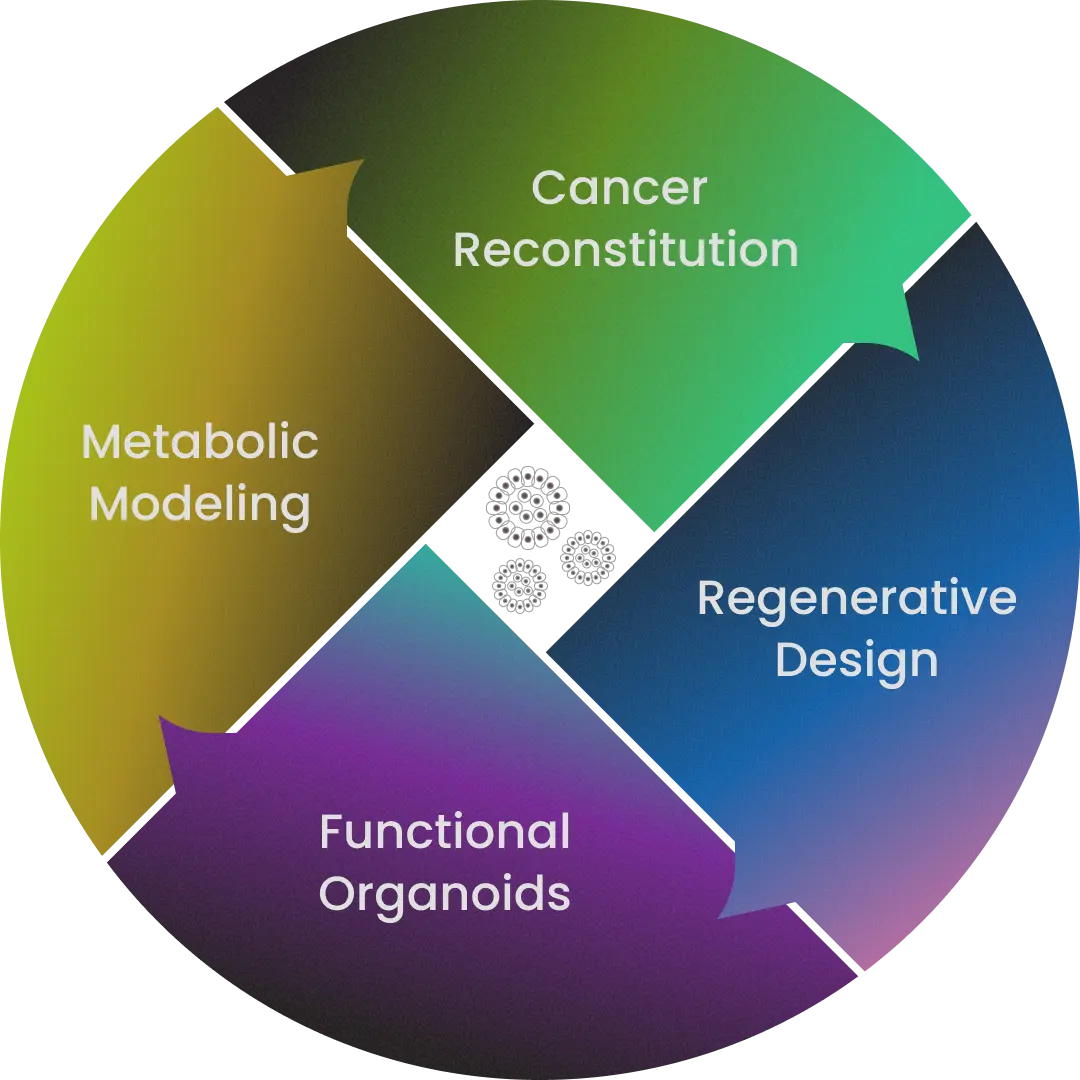
To uncover the principles behind this transition from health to disease, we use organoid technology—three-dimensional, miniature organ-like structures grown from a single human cell. Building on this platform, our work centers on four pillars:
Cancer Reconstitution Recreating the "Natural History" of Cancer to Deepen Understanding
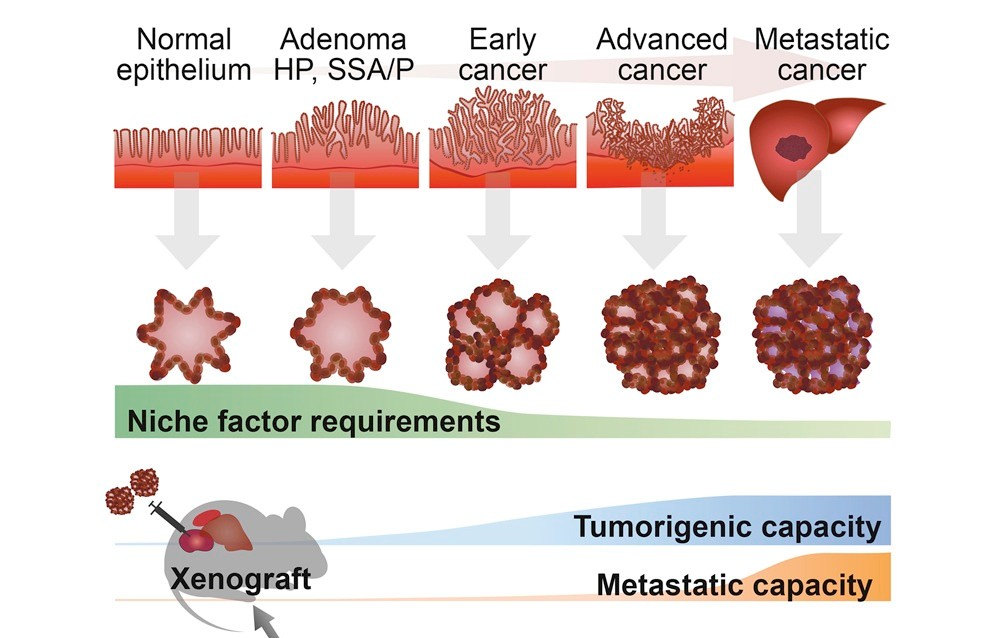
Cancer arises as genetic and epigenetic alterations accumulate over years, gradually transforming normal stem cells into malignant ones. By establishing cancer organoids—three-dimensional cultures derived from human tumor tissues—we can reconstruct how cancers emerge and progress, as if retracing their natural history inside the body.
With the integration of gene-editing technologies, cancer organoids now allow us to probe processes that were once nearly inaccessible: how invasion and metastasis unfold, how cancers adapt under therapeutic pressure, and how resistance ultimately arises.
Key Research Directions
Can we replicate all clinical cancers in vitro?
Patient-derived cancer organoids (PDOs) can be stably expanded in three dimensions while faithfully retaining the histology and genetic landscape of the original tumor. To date, we have established over 1,000 PDO lines covering major cancers such as colorectal, gastric, pancreatic, and lung. These models provide unprecedented opportunities to dissect cancer heterogeneity in genetics, drug response, and malignancy.
We also take on the challenge of modeling rare and difficult-to-culture cancers. A portion of these resources is shared globally through the Organoid Consortium, supporting target discovery and drug development in collaboration with domestic and international partners.
Can we generate cancer from normal cells?
To tackle the “ultimate question” of whether defined sets of oncogenes alone can give rise to cancer, we conduct reverse-engineering experiments. By introducing precise mutations into normal organoids, we reconstruct the stepwise trajectory of tumorigenesis.
This is made possible by our optimized CRISPR-Cas9 toolkit for organoids. For example, in human colon organoids, we introduced multiple driver mutations implicated in colorectal cancer and genetically reconstituted tumorigenesis in vitro.
Why does cancer become lethal?
The fatality of cancer often stems from a minority of tumor cells with the capacity to initiate new tumors—cancer stem cells. They are the engines of invasion, metastasis, and therapy resistance.
Using organoid-based gene-editing and live imaging, we have begun to visualize and trace the behavior of these cancer stem cells, uncovering how they self-renew, how they evolve under selective pressures, and how they shape the malignant fate of the tumor.
Regenerative Design Rebuilding Organs with Organoids
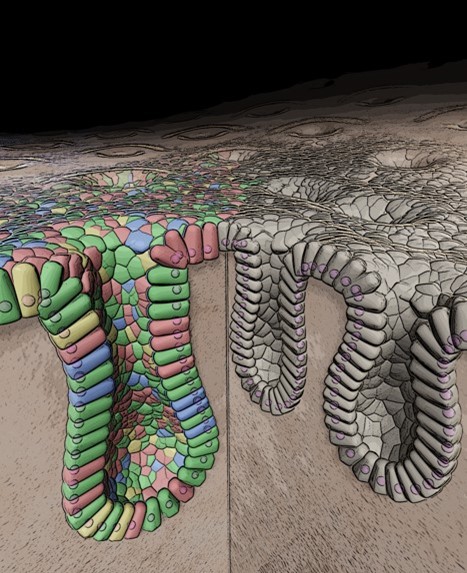
The intestine is one of the most dynamic organs in the human body: its epithelial lining completely renews itself within just a few days. Traditionally, how the intestine repairs itself after injury caused by inflammation or trauma has been studied only in mouse models. To overcome this limitation, we have established human intestinal organoid systems that allow us to directly explore the mechanisms of regeneration in human tissues.
Key Research Directions
Can the colon be “reprogrammed” into the small intestine to compensate for lost function?
Because organoids can be cultured directly from human tissue stem cells, they hold promise for regenerative medicine. Our research is advancing toward therapies for difficult intestinal diseases, including ulcerative colitis—increasingly common among younger individuals and marked by impaired mucosal repair—and short bowel syndrome, where insufficient intestinal length leads to severe malabsorption.
We have developed a breakthrough method for transplanting normal human intestinal organoids into the intestines of immunodeficient mice. These transplanted organoids retained their human-specific traits, formed epithelial structures, and remained non-tumorigenic over the long term. Even more strikingly, we transplanted small intestinal organoids into the colon, generating a “small-intestinized colon” capable of absorptive functions. This offers a potential therapeutic strategy for short bowel syndrome and related disorders.
Together, these achievements represent an important step toward regenerative medicine powered by organoids—and ultimately toward First-in-Human clinical trials.
Functional Organoids Recapitulating Human Tissue Function
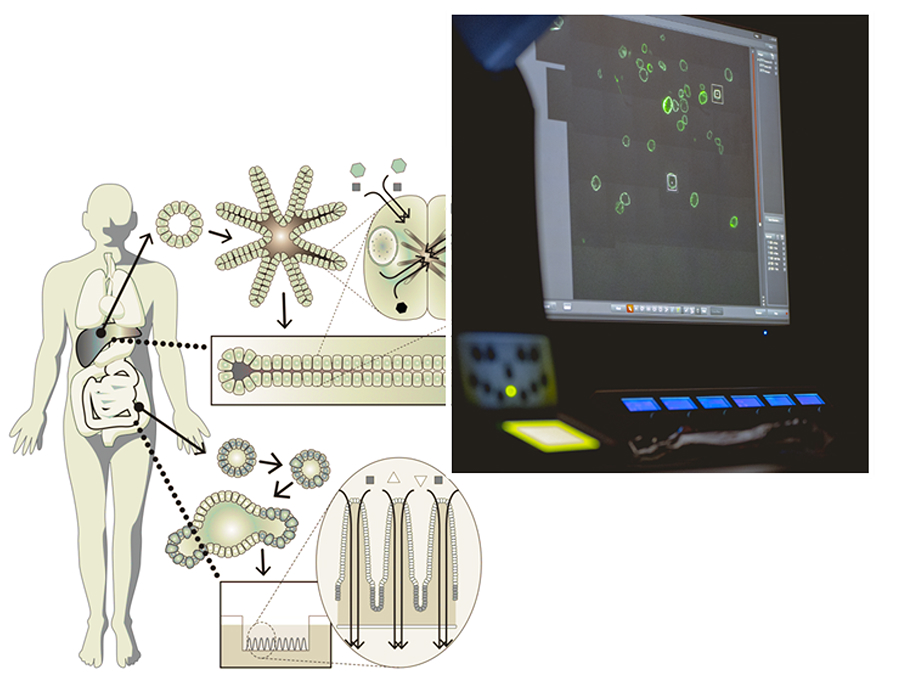
Organoid technology has transformed the way we study human tissues, and recent advances have even enabled organoids to be generated from induced pluripotent stem (iPS) cells. Yet, the word “organoid” still primarily refers to something that resembles an organ. To truly impact medicine, organoids must move beyond morphology and gene expression toward faithfully reproducing the functions of living tissues.
Key Research Directions
Do organoids that “look like” tissues also “function” like them?
For organoids to become genuinely functional, they must undergo functional maturation—including correct polarity, organelle organization, and protein localization. While conventional differentiation relies mainly on growth factor withdrawal, we are identifying new drivers of functional specialization to push organoid systems forward.
We are also building evaluation platforms that quantify organoid functionality across three axes: structure, metabolism, and signaling. By combining these dimensions, we aim to transform organoids from morphological mimics into fully functional models of human tissue.
Metabolic Modeling Illuminating the Invisible Metabolism
Metabolism lies at the very core of life. Inside cells, countless enzyme-driven reactions transform molecules into the energy and building blocks required for survival. These processes form intricate, highly organized networks that adapt to environmental, genetic, and chemical changes. Our laboratory seeks to recreate in vivo–like metabolic states in vitro, combining technologies such as metabolic imaging, mass spectrometry, and flux analysis.
Key Research Directions
Can metabolism be faithfully reproduced in vitro?
For drug toxicity testing and therapies for metabolic disorders, it is essential to model metabolism accurately in human tissues. Yet for decades, it has been extremely difficult to maintain long-term, functional hepatocytes in culture.
We have now developed organoid-based liver models that capture the essential metabolic functions of hepatocytes. Building on this success, we are extending the approach to other organs, opening new avenues for studying metabolism and developing treatments for metabolic disease.